FDA lowers Pfizer-BioNTech vaccine age to 12, holds virtual press conference
Amanda Hare - live snip from Zoom meeting
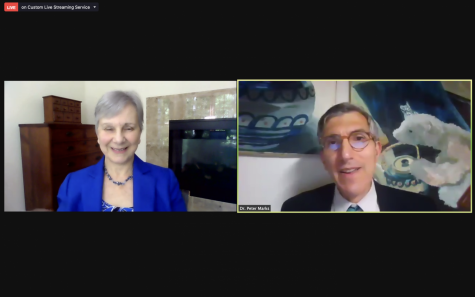
A screenshot shows Peter Marks, the Director of the Center for Biologics Evaluation and Research within the Food and Drug Administration, and FDA commissioner Janet Woodcock talking in a virtual press conference with student journalists on May 18. The press conference came after the FDA announce on May 12 that the Pfizer-BioNTech vaccine would now be available to children of ages 12 to 15. “The FDA will continue to work diligently to protect the health and safety of Americans of all ages and we’re committed to making decisions that are guided by science people can trust,” Marks said. “We know that working together, we can overcome the challenge of COVID-19.”
May 19, 2021
In another step to fight COVID-19, the Food and Drug Administration authorized the extended use of the Pfizer-BioNTech vaccine for children ages 12 to 15 on May 12. On Tuesday, May 18, the FDA held a virtual press conference for middle and high school student journalists to ask FDA commissioner Janet Woodcock and Peter Marks, the Director of the Center for Biologics Evaluation and Research within the FDA, questions about the announcement.
The FDA’s announcement expanded the emergency use authorization, or EUA, of the Pfizer-BioNTech vaccine from the age of 16 to the age of 12. After the announcement, the Center for Disease Control and Prevention’s advisory committee on immunization practices met to discuss the announcement voted to endorse this EUA. While PISD has been in school this year, many students have opted for virtual learning due to the pandemic. This development may make it easier for students to make the decision to return to in-person learning next year.
“The FDA has done everything we can to assure that the COVID-19 vaccines we’ve authorized have met our high standards for quality safety and effectiveness we know that every time someone, including members of our own family, receives a COVID-19 vaccine, they’re putting their trust in us,” Woodcock said during the May 18 virtual press conference. “We’re humbled by this trust, and we’re working hard every single day to maintain that trust as families consider the Pfizer-BioNTech COVID-19 vaccines for their kids.”
Since the announcement, more than 600,000 adolescents aged 12 to 15 years old have received the first shot of the vaccine. For more information, FAQs and fact sheets are available on the FDA website.
“To determine that the safety of the vaccine, the FDA looked at results from a clinical trial of 12 through 15 years old,” Marks said. “Half of the participants received the Pfizer-BioNTech COVID-19 vaccine and half received a placebo made of saline or saltwater.”
Researchers used a placebo to help them understand the effects a new drug or treatment could have on a particular condition. Some groups are tested under the real drug, and others only receive the placebo.
“More than half of the participants in the clinical trial were followed for safety for at least two months after they receive the second dose of the vaccine, and the side effects seen in those ages 12 through 15 were similar to those experienced by people 16 and older who received the vaccine in earlier clinical trials. But, some people experience side effects following any vaccination,” Marks said. “Everyone’s experience isn’t the same, and some might not experience any side effects at all.”
In the study, 1,005 children received the vaccine, and none of them contracted COVID-19. Of the 978 children who got the placebo, 16 of them were diagnosed with COVID-19. The most common side effects of the vaccine lasted one to three days and included pain at the injection site, tiredness, headache, chills, muscle pains, fever and joint pain. However, these effects are more common after the second dose of the vaccine than the first dose.
“There’s no difference in the way that Pfizer-BioNTech COVID-19 vaccine will be administered to 12 through 15 year olds,” Marks said. “It’s the standard series of two doses three weeks apart, using the same dose that’s given to those 16 years of age and older.”
As for what this change means for mask guidelines, Woodcock said it will still be up to “local jurisdictions.”
“There’s a lot of variability there,” Woodcock said. “We think that the response to this vaccine was very robust in the children who received it, and it seemed to protect them very well, but we don’t know in a large variety of people. There are individuals who may be immunocompromised or have some other condition that may not react so well. So, I think each jurisdiction will probably look to CDC guidance, to what the state is saying, to what the local community prevalence of the virus is circulating around and what kind of variants are circulating, and they’ll put all that together.”
Woodcock also said that it is more difficult to study the vaccine on children under the ages of 12 years old due to change in doses.
“It’s easy to forget because these development programs have been so successful that generally speaking, a vaccine development program may take seven years in the clinic,” Woodcock said. “We’ve been very careful, and we can only compress this so much. Younger children seem to have a different immune response when they get affected with this virus.”
As of right now, there are 15,353 total students in-person in the district with four active cases as of May 18. Next year, masks will be optional and virtual learning will still be available. These vaccination opportunities could increase the number of in-person students in the district as it may make students more comfortable to come in-person.
“Parents/guardians should have the right to make the best decision for their child concerning vaccination,” superintendent Holy Ferguson said.
Woodcock said this is a “big step” and will bring everyone closer to “returning to a sense of normalcy and ending the pandemic.”
“Through April 2021, approximately 1.5 million children 11 through 17 years old were diagnosed with COVID-19,” Woodcock said. “Reopening our schools and communities is top of mind for many parents and kids. Getting our children vaccinated to prevent the disease will help our children have families get back to a more normal routine. If you’re now eligible for COVID-19 vaccine, I encourage you to talk to your parents about getting your vaccine and talk to your friends about getting theirs.”
Editor’s Note: Special thanks to 2021-2022 Eagle Nation Online Editor in Chief Amanda Hare for attending this May 18 live press conference, sponsored by the Journalism Education Association exclusively for scholastic journalists.
This story was originally published on on May 19, 2021.

























![IN THE SPOTLIGHT: Junior Zalie Mann performs “I Love to Cry at Weddings,” an ensemble piece from the fall musical Sweet Charity, to prospective students during the Fine Arts Showcase on Wednesday, Nov. 8. The showcase is a compilation of performances and demonstrations from each fine arts strand offered at McCallum. This show is put on so that prospective students can see if they are interested in joining an academy or major.
Sweet Charity originally ran the weekends of Sept. 28 and Oct. 8, but made a comeback for the Fine Arts Showcase.
“[Being at the front in the spotlight] is my favorite part of the whole dance, so I was super happy to be on stage performing and smiling at the audience,” Mann said.
Mann performed in both the musical theatre performance and dance excerpt “Ethereal,” a contemporary piece choreographed by the new dance director Terrance Carson, in the showcase. With also being a dance ambassador, Mann got to talk about what MAC dance is, her experience and answer any questions the aspiring arts majors and their parents may have.
Caption by Maya Tackett.](https://bestofsno.com/wp-content/uploads/2024/02/53321803427_47cd17fe70_o-1-1200x800.jpg)
![SPREADING THE JOY: Sophomore Chim Becker poses with sophomores Cozbi Sims and Lou Davidson while manning a table at the Hispanic Heritage treat day during lunch of Sept 28. Becker is a part of the students of color alliance, who put together the activity to raise money for their club.
“It [the stand] was really fun because McCallum has a lot of latino kids,” Becker said. “And I think it was nice that I could share the stuff that I usually just have at home with people who have never tried it before.”
Becker recognizes the importance of celebrating Hispanic heritage at Mac.
“I think its important to celebrate,” Becker said. “Because our culture is awesome and super cool, and everybody should be able to learn about other cultures of the world.”
Caption by JoJo Barnard.](https://bestofsno.com/wp-content/uploads/2024/01/53221601352_4127a81c41_o-1200x675.jpg)












